Jason S. Lee1 and Brenda J. Little2 1Naval Research Laboratory, Stennis Space Center, MS, USA 2B.J. Little Corrosion Consulting, Montgomery, TX, USA Low alloy steel pipelines, used to produce, gather, transport, and distribute crude oil, refined/blended petroleum products, and natural gas, are located in a wide variety of microbiologically active environments, including soils, marine and estuarine waters, and undersea sediments [1]. Microbiologically influenced corrosion (MIC), caused by the presence and activities of microorganisms associated with biofilms, is an acknowledged problem for internal and external pipeline surfaces. Statistics related to the prevalence of corrosion in oil and gas (O&G) pipelines and more specifically to MIC are confusing. Most of the statistics, originally established for particular facilities, are now cited in more general contexts. The result is that the significance of the statistics is lost in the overgeneralization. In 2002, the National Association of Corrosion Engineers (NACE) International (now The Association for Materials Protection and Performance [AMPP]) [2] estimated the cost of corrosion for onshore gas and liquid transmission pipelines was $7 billion. There were no specific statistics related to MIC. In 2012, the Department of Transportation (DOT) statistics [3] suggested that 16 and 7% of releases of crude oil in the United States are due to internal and external and corrosion, respectively. When reviewing MIC in the O&G sector in general, Wolodko et al. [4] reported, “…almost 40% of internal and 20–30% of external corrosion could be attributed to MIC.” The authors cite Kaduková et al. [5]. Kaduková et al. [5] quoted the same estimates but attributed the percentages to Rajasekar et al. [6] and Beavers and Thompson [7]. Rajasekar et al. [6] stated, “… 40% of all internal pipeline corrosion in the gas industry (emphasis added by the authors) can be attributed to microbial corrosion,” Graves and Sullivan [8]. The original work by Graves and Sullivan [8] was specific for gas gathering pipelines originating from gas wellheads. Beavers and Thompson [7] evaluated pipeline data from 1994 to 1999 and reported that 36% of accidents in natural gas transmission pipelines were due to external corrosion, while 63% were caused by internal corrosion. For natural gas distribution pipeline accidents, approximately 4% were the result of corrosion, predominantly external corrosion. Statistics were not reported for MIC. Unlike the gas wells drilled in the 1990s, in the 2020s, O&G wells are not typically identified as separate facilities and both products are recoverable for a single well. However, because internal corrosion due to MIC in O&G pipelines is directly related to the operating conditions and biodegradability of the product(s) transported in the pipeline, any statistic related to MIC should include pipeline specifics. The following sections provide new insights into the complexity of microbial interactions leading to MIC of carbon steel and the need for approaches beyond the identification and enumeration of specific groups of microorganisms. The following sections will be limited to MIC of low alloy steels, i.e., carbon steels. Pope [9] suggested that craters, pits within pits, striations within pits, and tunneling were consistent with MIC in carbon steel gas pipelines. The critical alloying element in carbon steel is carbon and its mechanical properties depend on the percentage of carbon. However, carbon content has little effect on the general corrosion resistance [10]. Low-carbon steel contains approximately 0.05–0.3 wt% carbon and mild carbon steel contains 0.3–0.6% carbon. In referencing the work of others in this chapter, the alloy terminology used in the original work will be maintained. MIC-causing microorganisms are from all three main branches of evolutionary descent, that is, bacteria, archaea (methanogens), and eukaryota (fungi). Some microorganisms are naturally occurring in hydrocarbon fuels; others are introduced from air or water. Oil reservoirs provide conditions for the proliferation of bacteria and archaea, such as sulfate-, nitrate-, and iron-reducers, fermenters, acetogens, and methanogens [11]. The list of microorganisms involved in MIC and the mechanisms by which they influence corrosion is continuously growing. Mechanisms are the result of specific metal/microbe/electrolyte interactions. Corrosion is directly related to oxidation (anode) and reduction (cathode) reactions and microbial processes require one- and two-electron transfers (either oxidation or reduction reactions). Microorganisms can accelerate rates of partial reactions in corrosion processes or shift the mechanism for corrosion. MIC of carbon steel can involve a conversion of a protective metal oxide to a less protective layer (e.g., a sulfide) or removal of the oxide layer, for example, by metal oxide reduction or acid production. Sulfide layers are easily detached by mechanical sheer, resulting in enhanced erosion corrosion. Under specific environmental conditions, MIC can result from the direct uptake of electrons from metallic (zero valence) iron (Fe0). On carbon steel, microorganisms can produce localized attack, including pitting, galvanic corrosion, stress corrosion cracking, and hydrogen embrittlement. The microorganisms typically identified with MIC are based on specific metabolisms, e.g., sulfate reduction, iron reduction, slime formation, iron and manganese oxidation, and methanogenesis. Long lists of microorganisms with “corrosive” metabolisms have been compiled [12]. However, the classification of MIC-related microorganisms by a single metabolic function can be misleading [13]. Because the microorganisms typically associated with MIC are metabolically versatile, they can express multiple pathways other than those commonly attributed to them. Vigneron et al. [13] summarized the findings of several investigations by suggesting that corrosive biofilms contain numerous microhabitats with different redox potentials and chemical gradients, allowing the establishment of microorganisms with parallel, complementary, and antagonistic physiologies (Figure 55.1). The authors concluded that most microorganisms in corrosive biofilms have either a direct or indirect effect on corrosion [14]. Figure 55.1 Conceptual model (not to scale) of microbial communities and processes involved in MIC of the tube section. Each microbial group was characterized by their potential metabolic functions: fermentation, iron oxidation, iron reduction, sulfate reduction, elemental sulfur reduction, and biofouling. (Adapted from Vigneron et al. [13]. Copyright © 2018, Springer-Verlag GmbH Germany, part of Springer Nature.) Microorganisms have developed several strategies for survival in natural environments: (1) biofilm formation, (2) spore formation, (3) dwarf cells, and (4) a viable, but nonculturable state. Microorganisms can form biofilms on interior and exterior surfaces of pipes. Biofilms contain living and dead cells enmeshed in a matrix of extracellular polymeric materials, e.g., exopolysaccharides. Universal properties of undisturbed biofilms are horizontal stratification of electron acceptors with depth [15] and alteration of surface chemistries that may lead to corrosion. Many filamentous fungi and some bacteria produce spores that are resistant to temperature, acids, alcohols, disinfectants, drying, freezing, and other adverse conditions. Spores may remain viable for hundreds of years and can germinate when conditions become favorable. However, there is a difference between survival and growth. Growth of the causative organisms requires water, energy sources (i.e., electron acceptors and donors), and nutrients. The following discussion of growth requirements does not account for the complexity of complementary syntrophic interactions within biofilms, where one microbial species lives off the products of another species. The potential for MIC is determined by the availability of these essentials and any proposed mechanism must account for their availability. Liquid water is a requirement for all forms of life. Some water can be dissolved in oil but beyond the saturation point water is either emulsified or free. Microbial growth in hydrocarbons is concentrated at oil/water interfaces, that is, emulsified water and separate water phases. Many oil- and gas-bearing rocks contain interstitial and connate waters. Pannekens et al. [11] suggested that the concept of an oil/water interface in oil reservoirs should be expanded to include pore water within the formation rocks and thin water films covering rock surfaces. Since water is a product of the microbial mineralization of organic substrates, it is possible for in situ microbial mineralization of a hydrocarbon to generate a water phase that can be used for further proliferation. Pannekens et al. [11] list other potential sources of water and extraneous microorganisms, e.g., drilling, flooding, hot steam, and water injections, e.g., seawater, fresh water, and recycled formation water. Water injections are required to increase reservoir pressure in secondary oil production. The amount of water in an oil plays an important role in microbial processes. Oil samples with 40–60% water content harbored a 2.6-fold higher bacterial richness compared to oils with 1–5% [11]. Richness refers the total number of diverse species. Microorganisms obtain energy through electron transfer processes. Not all electron donors and acceptors are water soluble. Electrogenic bacteria are capable of moving electrons to and from solid materials. Petroleum hydrocarbons, organic matter, reduced inorganic compounds, molecular hydrogen, and iron can act as electron donors. Electrons are then channeled to electron acceptors. During this process, the electron donor is oxidized and the electron acceptor is reduced. Microorganisms can use various electron acceptors for respiration in dissimilatory reactions, that is, the acceptors are not assimilated. In aerobic respiration, energy is derived when electrons are transferred to oxygen, the terminal electron acceptor. In anaerobic respiration, various organic and inorganic compounds may be used as terminal electron acceptors, including sulfate, carbon dioxide, nitrate, nitrite, chromium (VI), iron (III) (ferric ion), and manganese (IV). There is specificity among anaerobes for electron acceptors; bacteria are routinely grouped based on the terminal electron acceptor in anaerobic respiration, for example, sulfate-, nitrate-, and metal-reducing bacteria. Facultative anaerobic bacteria can use oxygen or other electron acceptors. Obligate anaerobic microorganisms cannot tolerate oxygen for growth and survival. Obligate anaerobic bacteria and archaea are, however, routinely isolated from oxygenated environments associated with particles, crevices, and most importantly, in association with aerobic and facultative bacteria that effectively remove oxygen from the immediate vicinity of the anaerobe. Sulfate reducing bacteria (SRB) are the microorganism most frequently identified with MIC of interior and exterior O&G assets. SRB activity reportedly causes the average corrosion rate of steel exposed to soil in the absence of oxygen to be >20 times higher than that of abiotic controls [16]. SRB are a group of ubiquitous, diverse anaerobes that use sulfate as the terminal electron acceptor, producing hydrogen sulfide (H2S). Several SRB can also reduce nitrate, sulfite, or thiosulfate. Under specific conditions, some SRB can accept electrons directly from Fe0 and transfer the electrons for sulfate reduction. Enning et al. [17] demonstrated direct uptake of electrons from Fe0 through a semiconductive iron (II) (ferrous ion) sulfide corrosion crust. Many archaea can also produce sulfides. The inclusive term for all sulfide-producing microorganisms is sulfide-producing prokaryotes (SPP). Several corrosion mechanisms have been attributed to SPP, including cathodic depolarization by the enzyme dehydrogenase, anodic depolarization, production of iron sulfides, release of exopolymers capable of binding metal ions, sulfide-induced stress corrosion cracking, and hydrogen-induced cracking or blistering [18]. During corrosion of carbon steel influenced by SPP, a thin (approximately 1 μm), adherent layer of mackinawite (tetragonal iron1+x sulfide (Fe1+xS) (x = 0–0.11)) is formed. If the ferrous ion concentration is high, mackinawite and green rust 2, a complex ferrosoferric oxyhydroxide will form. Under some circumstances, green rust 2, unstable in the presence of oxygen, can be an electron acceptor for SRB [19]. Once electrical contact is established between corrosion products and carbon steel, the carbon steel behaves as an anode and electron transfer occurs through the iron sulfide. In the absence of oxygen, the metabolic activity of SPP causes accumulation of H2S near metal surfaces. At low ferrous ion concentrations, adherent and temporarily protective films of iron sulfides form on low-alloy steel surfaces with a consequent reduction in the corrosion rate. High rates of SPP-induced corrosion of carbon steel are maintained only when the concentrations of ferrous ions are high. In the absence of oxygen, sulfides, from whatever source, react with carbon steel to from a layer of iron sulfide that prevents further reaction, that is, diminution of corrosion. Aggressive SPP corrosion of low alloy steel has been reported in the presence of dissolved oxygen. Hardy and Bown [20] investigated the weight loss of mild steel exposed to successive aeration–deaeration shifts. In their experiments, the highest corrosion rates were observed during periods of aeration. In laboratory seawater/hydrocarbon fuel incubations, Aktas et al. [21] demonstrated that there was minimal sulfate reduction and no corrosion of carbon steel in the total absence of oxygen. Aggressive corrosion was observed when low levels of dissolved oxygen (<100 parts per billion) were present in the seawater. Hamilton [22] reviewed mechanisms for MIC and concluded that oxygen was the terminal electron acceptor in many MIC reactions. Following this logic, when SPP are involved in corrosion, sulfate could serve as the terminal electron acceptor in respiration, but oxygen will be the terminal electron acceptor in the corrosion reaction. Assimilable forms of carbon, nitrogen, phosphorus, and sulfur are required to support microbial growth. Hydrocarbons can be degraded under aerobic and anaerobic conditions to provide carbon sources [23–28]. Aerobic biodegradation of hydrocarbons is faster than anaerobic degradation. Rates depend on the specific electron acceptors used in the process, e.g., molecular oxygen > nitrate > ferric ion > sulfate > carbon dioxide. As a practical matter, carbon availability is not typically the main constraint for microbial growth in pipelines. One cannot make assumptions about nutrients or growth-limiting factors in soils by measuring elemental composition of a soil extract or by assuming that all nutrients in soil are soluble. Some soils are deficient in soluble phosphate but high in insoluble mineral phosphates and phosphate-solubilizing microorganisms. Many soil microorganisms can fix nitrogen (N2) enriching the soil in nitrogen-rich compounds, e.g., ammonia, nitrates, or nitrites. O&G pipelines are classified by function. Production and gathering pipelines are considered to perform the same function for O&G wells, but their contents can be different. Production lines are the pipes and equipment located near the wellhead. Natural gas gathering systems gather the raw material, methane, mixed with water, sulfur, and other gases (e.g., ethane, propane, and butane) and deliver to a facility for further refinement or to transmission pipelines. Gathering pipelines for oil production collect products from wells, tankers, or other pipelines and can contain crude oil, water, and mixed gases. Gases and fluids may be separated in gathering lines or may remain comingled until refined. Transmission pipelines are large diameter pipelines that transport gas or oil across large distances at high pressure. Once petroleum is refined into products, e.g., gasoline or kerosene, the product is transported via pipeline systems to storage or distribution stations. Distribution systems distribute products to homes, businesses, and storage facilities. Internal corrosion due to MIC is directly related to the biodegradability of the contents as well as the availability of water and electron acceptors/donors. Water in fuel systems can contribute nutrients in two ways. Some fuel components are soluble in water. In addition, a separate water phase allows an interface where biodegradation can take place. Susceptibility of hydrocarbons to microbial degradation can be generally ranked as follows: linear alkanes > branched alkanes > small aromatics > polyaromatics > cyclic alkanes. The sulfur content of crude oils is a particular concern from a MIC perspective because SPP could use oxidized sulfur compounds, including sulfate, as electron acceptors to produce H2S. However, in past surveys, sulfur content did not correlate to H2S content [29]. Most of the sulfur in crude oils is organic sulfur in heterocyclic ring structures, for example, polycyclic saturated carbonaceous ring structures. Gogoi and Bezbaruah [30] concluded, “… most prevalent naturally occurring microorganisms do not effectively breakdown sulfur-bearing heterocycles,” suggesting that these compounds are not readily biodegradable. Low concentrations of assimilable forms of nitrogen and phosphorus can limit hydrocarbon biodegradation. However, both may be added in pipelines as corrosion inhibitors (i.e., nitrates and phosphates). Additions of biofuels, i.e., fuels produced from renewable organic materials, can introduce biodegradable components to blended petroleum products. During production, oil is pumped to the surface as a mixture of water, oil, and gas. Ciaraldi et al. [31] concluded that the factors influencing MIC in production lines in the Gulf of Suez were low flow velocities, deposit accumulations, water flooding, and increased levels of bacteria. Vigneron et al. [13, 14] reported that piping within crude oil offshore production facilities was particularly vulnerable to MIC because of the continuous supply of anaerobic nutrient-rich produced water combined with petroleum hydrocarbons, organosulfur compounds (e.g., benzothiophenes), volatile fatty acids, and other end products of fermentation at ambient temperatures (15–35 °C). Low velocity flow in production lines allows sediment and bacteria to settle. Salgar-Chaparro et al. [32] used multispecies biofilms from two oilfield consortia to demonstrate the impact of nutrients from produced water on biofilm development and corrosivity. Microorganisms grown under nutrient replenishment conditions produced thicker, more active biofilms, and greater localized corrosion on carbon steel. Flow reactors with a continuous flow of nutrients, the equivalent of piping in which produced fluids are normally flowing, produced more localized corrosion than reactors operated under batch conditions, the equivalent of piping maintained with intermittent flow. Vigneron et al. [13, 14] used multiple techniques to identify microorganisms from the surfaces of failed pipes, including complementary molecular approaches coupling quantitative polymerase chain reaction (qPCR), ribosomal intergenic spacer analysis, and multigenic DNA next-generation sequencing. Bacterial and archaeal 16S rRNA gene diversity was complemented by sequencing of the dsrAB and mcrA genes, which code for key enzymes in sulfate reduction and methanogenesis, respectively. Sulfate-, elemental sulfur-, and iron-reducing microorganisms were predominant in the biofilms and the authors concluded that they were important contributors to the high corrosion rate and corrosive by-products. However, authors suggested that other microorganisms, not typically identified with MIC, with fermentative metabolisms or production of exopolysaccharides, might provide a favorable environment for microbial growth and corrosion. Both free and emulsified water must be reduced to specified levels before oil can be sold and transported in pipelines. However, there is recognition that some emulsified water is transferred into pipelines. Petroleum transmission lines are less susceptible than production lines to MIC because oxygen, water, and sediment are removed to specified limits. For example, in Canada, the National Energy Board (NEB) requires that crude transmission pipelines cannot accept a product that contains more than 0.5% basic sediment and water (BS&W). Lillebo et al. [33] demonstrated that the growth of SRB was inhibited in crude oils containing <0.5% water. In the United States, the Federal Energy Regulatory Commission (FERC) allows BS&W levels of 1.0%. At these low levels, the water in crude oil exists as a microemulsion, resulting in carbon steel surfaces being oil-wetted and corrosion is negligible. BS&W values are averaged readings, meaning that it is possible to have slugging events that are not detected. Despite the potential differences in the water content, Friesen et al. [34] demonstrated there was no direct relationship between water content of crudes and corrosivity. Furthermore, internal corrosion has been observed in crude pipelines with <0.5% BS&W at locations where water can accumulate [35, 36]. Unintentional introduction of water or oxygen into crude oils increases the likelihood for MIC. Papavinasam [36] concluded that “bulk crude oil may indirectly affect the corrosion by influencing the locations where water accumulates, by influencing the type of emulsion, by impacting the wettability of phases on the steel surface and by supplying chemicals that can partition into the water phase.” Accumulation of water depends on inclination of the pipe, the flow velocity, and the cleanliness of the pipeline. Water solubility increases with hydrocarbon molecular weight [37]. However, industrial experience indicates that heavier crudes, while high in water content, are less corrosive owing to their elevated viscosity and resulting low conductivity (<10−7 S/cm) [37]. Asphaltenes and resins in heavy crudes act as surfactants to stabilize water-in-oil emulsions. Crudes carry water-wetted particles that can drop out at locations downstream of over-bends. Sludge deposits concentrate water from oil at the pipeline surface shifting the oil-wet surface to a water-wet surface. Sludge deposits are combinations of hydrocarbons, sand, clay, corrosion products, and biomass that can reach 50% water by weight. Mosher et al. [35] demonstrated that high bacterial activity and/or water content in the sludge alone did not produce corrosive conditions over a 3-month period. Analyses of pipeline deposits obtained from pigging operations indicated a range of particle sizes with diameters from 44 to 400 μm. Most of the solids were fine particles of silica sand and iron minerals. Larger sand particles were uniformly coated with very fine clay surrounded by a film of water. Under low flow conditions, these particles precipitate and form a sludge deposit. Gaylarde et al. [38] concluded that the major microbial problems in the petroleum refining industry were contamination of stored products, which leads to, “… loss of product quality, formation of sludge and deterioration of pipework and storage tanks, both in the refinery and at the end-user.” Passman [39] reviewed changes in formulations and additives that increased biodeterioration risks for fuels and fuel systems. Sources of free water in storage facilities include rain, water of condensation, and accumulated water from previous fuel additions. Gasoline contains straight and branched hydrocarbons, alkenes, naphthalenes, aromatics and stabilizers, and corrosion inhibitors. As a result of environmental regulations, oxygenated components have been blended into gasoline, e.g., alcohols (ethanol or methanol) and ethers (methyl tertiary-butyl ether or ethyl tertiary-butyl ether). The Renewable Fuel Standard (Energy Policy Act, 2005) and the Energy Independence and Security Act (2007) mandated increase in the production of “clean renewable fuels,” e.g., ethanol. Subsequently, there was an increase in retail outlets storing and dispensing 10% fuel-grade ethanol (FGE) 90% gasoline blends (also known as E10). Williamson et al. [40] concluded that low water availability could inhibit microbial activity under ideal operating conditions in E10 storage and transportation systems. However, microbial conversion of ethanol to acetic acid could potentially cause MIC of carbon steel (e.g., tanks, pipes, and pump sumps) in which FGE and water were present. Sowards et al. [41] measured the effects of microbial acetic acid and hydrogen sulfide production on fatigue-crack growth of two pipeline steels (API 5L X52 and X70). Microbiological species were responsible for significant increases in fatigue-crack growth rate across a wide range of amplitudes. The authors concluded that the mechanism for increased fatigue damage was hydrogen uptake through adsorption into the steel, which embrittles material ahead of the growing fatigue crack. Carbon steel has not required any specific changes to accommodate the storage of reformulated and oxygenated (ethanol and methanol) fuels [42]. Jet propulsion (JP) fuels, e.g., JP-5, JP-8, and Jet A, are kerosene-based fuels containing more than 200 aliphatic and aromatic hydrocarbons (C6-C17+). Aviation fuels are refined by a straight distillation of crude/shale oil. Performance-enhancing additives may include antioxidants; inhibitors for static, corrosion, and icing; and lubrication and thermal stability improvers and biocides. Additive concentrations are specified by commercial and military regulations. The relationship between MIC and JP fuels has been studied extensively in aluminum fuel tanks [43, 44]. Increased incidence of biodeterioration problems in carbon steel aviation storage tanks in the early 2000s coincided with the replacement of ethylene glycol monomethyl ether, a toxic icing inhibitor, with less toxic diethylene glycol monomethyl ether. The switch caused a shift in the microbial community from predominantly fungal to bacterial contaminants. Jet fuel requires storage in epoxy lined steel tanks [45]. The authors of this chapter are unaware of any reports of MIC in those tanks. Sustainable aviation fuel (SAF) derived from renewable biomass is certified for commercial use and can currently be blended up to 50% with traditional jet fuel without modifications to airplanes, engines, or fueling infrastructure [46]. Use of SAF produces lower carbon emissions compared to the traditional jet fuel. The International Energy Agency’s Sustainable Development Scenario predicts that biofuels will meet 10% of aviation fuel demand by 2030, and approximately 20% by 2040 [47]. Petroleum diesel fuel is a hydrocarbon product with carbon chain lengths of C15–C22. Since 2000, there have been major sequential and concurrent changes in the chemistry of diesel in the United States. Additives, e.g., aliphatic amines, chelating agents, detergents, and corrosion inhibitors, have been used to improve the stability. In 2006, the United States Environmental Protection Agency (EPA) Clean Air Highway Diesel Final Rule mandated a 97% reduction in sulfur content of highway diesel fuel. Sulfur content was reduced from 500 parts-per-million (ppm) in low sulfur diesel (LSD) diesel fuel to 15 ppm in ultra-low sulfur diesel (ULSD). Reports of severe and accelerated corrosion of metal parts associated with storage tanks and dispensing equipment started in 2007. ULSD has lower fuel efficiency, lower lubricity, and lower water solubility than traditional diesel. Lower water solubility increases the susceptibility to water separation, corrosion, and microbial contamination. Fatty acid methyl ester (FAME) fuel production was increased in response to the Energy Independence and Security Act (2007). FAME fuels were added to ULSD, creating biodiesel. Biodiesel blends are designated Bxx where xx specifies the percentage of biodiesel. In the same year, the United States Air Force (USAF) adopted B20 (20% biodiesel and 80% petroleum diesel fuel) for use in nontactical support vehicles. Use of B20 correlated with increased reports of “bad fuel.” Stamps et al. [48] conducted an in situ study of microbial communities in underground storage tanks (UST). They linked proliferation of filamentous fungi and acid-producing bacteria (APB) to acetic acid production and pitting corrosion in actively operating B20 UST. Fungi and APB can increase corrosion rates of carbon steel when grown on ULSD [49]. However, because biodiesel is more hygroscopic and oxidatively unstable compared to petroleum-based diesel, fuel blends containing biodiesel are more susceptible to microbial degradation. Microorganisms can grow more rapidly and produce more biomass on the FAME in biodiesel blends than in ULSD [50]. The result is greater acid production and corrosion risk to infrastructure storing or dispensing fuels containing biodiesel. In 2012, the Clean Diesel Fuel Alliance (CDFA) [51] completed a study of rapid and severe corrosion in UST storing diesel and results were summarized in a 2016 EPA report [52]. CDFA hypothesized that ethanol, present in 90% of 42 samples, was a common contaminant of ULSD during fuel transportation. The reports suggest that in the presence of water and oxygen, ethanol could be oxidized to acetic acid, resulting in both aqueous and atmospheric corrosion. Passman [39] concluded that ULSD was more susceptible to biodeterioration than higher sulfur fuels because of reduced concentrations of complex, toxic polynuclear aromatic compounds. The Energy Institute (2019) suggested that the reduction of sulfur and the introduction of (FAME) fuel to diesel were introduced at roughly the same time, i.e., 2007, and that the individual influences on microbial contamination and MIC cannot be differentiated. The negative properties of ULSD are addressed with additives, e.g., corrosion inhibitors and lubricity enhancers. Biodiesel typically requires separate storage and handling facilities because of the tendency to gel in cold temperatures. Biodiesel is transported in the United States primarily by rail, truck, or barge shipment. Magellan Midstream Partners have tested the transport of batches of B5 through its transportation pipeline system [53]. Both biodiesel and renewable diesel can be produced from vegetable and animal oils. However, renewable diesel is produced through refinery hydrotreatments and can be stored and distributed with existing infrastructure. Renewable diesel does not require blending. One of the first attempts to quantify microorganisms related to MIC in O&G systems was published in 1975 by the American Petroleum Institute (API) [54], “Recommended Practice (RP) 38 Biological Analysis of Subsurface Injection Waters”. The RP describes liquid media for cultivation of SRB and heterotrophic bacteria. The RP states that the presence of SRB is “a potential problem.” It further states, “The extent of the problem will depend upon additional evidence ….” In 1990, the Gas Research Institute (GRI) published “MIC: Methods of Detection in the Field” [9]. The GRI guide “designed to help gas industry personnel determine whether or not the corrosion occurring at a particular site is MIC …” was the first to emphasize the importance of APB to the corrosion of carbon steel gas pipelines. The guide also specified localized corrosion morphologies that were suggestive of MIC, including cup-type, scooped-out hemispherical pits, and striation lines. The guide provided a numerical rating for predicting the probability that MIC had occurred based on two parameters, that is, the number of bacteria and the characteristics of pit morphology. The guide did not suggest using either parameter independently to diagnose MIC. The guide was not meant to be a predictive tool. Unintended consequences of the guide [9] were the proliferation of liquid media test kits, a strong reliance on numbers of particular types of bacteria to diagnose and predict MIC, and an overinterpretation of pit morphology to diagnose MIC [55]. Updated in 2014, NACE International Test Method (TM) TM0194-2014 “Field Monitoring of Bacterial Growth in Oil and Gas Systems” [56] provided sampling procedures for planktonic and sessile bacteria, a lactate-based culture medium, and a serial dilution to extinction methodology for enumerating SRB. Vials that turned black due to the formation of iron sulfide within a 28-day period were scored as positive for SRB. In addition, the time required for blackening was suggested as a measure of the “strength (i.e., activity) of the growing culture.” There is an acknowledgment within TM0194-2014 that the lactate-based medium cannot be used to grow SRB requiring other carbon sources, for example, acetate, propionate, or butyrate. When SRB growth in the recommended lactate-containing medium is unexpectedly low, the TM suggests screening with other culture media. Several attempts have been made to improve liquid culture media used for the detection of SRB. A complex medium was developed containing multiple carbon sources that could be degraded to both acetate and lactate [57]. In comparison tests, the complex medium produced higher counts of SRB from waters and surface deposits among five commercially available media [58]. Jhobalia et al. [59] developed an agar-based culture medium for accelerating the growth of SRB. The authors noted that over the sulfate concentration range from 1.93 to 6.50 g/l, SRB grew best at the lowest concentration. Cowan [60] developed a rapid culture technique for SRB based on rehydration of dried nutrients with water from the system under investigation. The author claimed that using system water reduced the acclimation period for microorganisms, ensuring that the culture medium had the same salinity as the system water used to prepare the inoculum. Cowan [60] reported quantification of SRB within 1–7 days. The distinct advantage of culturing techniques to detect specific microorganisms is that low numbers of viable cells can grow to easily detectable higher numbers in the proper culture medium. Under all circumstances, culture techniques underestimate the organisms in a natural population [61, 62]. Kaeberlein et al. [63] suggested that 99% of microorganisms from the environment resist cultivation in the laboratory. The actual percentage of microorganisms that can be cultured from an environmental sample depends on the culture medium composition and incubation conditions (e.g., temperature) and the relevance of both to the environment from which the sample was collected. A major problem in assessing microorganisms from natural environments is that viable microorganisms can enter into a nonculturable state [42]. Another problem is that culture media cannot approximate the complexity of a natural environment. Growth media tend to be strain specific. As previously mentioned, lactate-based media sustain the growth of lactate oxidizers but not acetate-oxidizing bacteria. Incubating at one specific temperature is further selective. Zhu et al. [64] demonstrated dramatic changes in the microbial population from a gas pipeline after samples were introduced into liquid culture media. For example, using culture techniques, SRB dominated the microflora in most pipeline samples. However, using culture-independent qPCR techniques, they found that methanogens were more abundant in most pipeline fluid samples than denitrifying bacteria and that SRB were the least abundant bacteria. Similarly, Romero et al. [65] used molecular monitoring to identify bacterial populations in a seawater injection system. They found that some bacteria present in small amounts in the original waters were enriched in the culture process. It is well established that the microbial constituents in sessile populations (attached to the surface, i.e., biofilms) are different from those of planktonic population (passively floating). Wrangham and Summer [66] used metagenomic analyses of planktonic and sessile samples from three different geographical locations to demonstrate that the planktonic population was not representative of the sessile population from the same location. They reported, “… planktonic and sessile populations from the same location may be as different from each other as they are to samples obtained from other locations.” Similarly, Larsen et al. [67] used molecular microbiological methods (MMM) to demonstrate that the bacteria in scale (corrosion product/biofilm) and produced water were “somewhat different” from each other. More recent test methods, for example, NACE TM0212-2018 “Detection, Testing, and Evaluation of MIC on Internal Surfaces of Pipelines” [68] acknowledge that many types of microorganisms, including archaea, can contribute to MIC. In section 7.2.4, the TM clearly indicates that the type of medium used in liquid culture techniques determines, to a large extent, the numbers and types of microorganisms that grow. In addition to liquid culture, the document describes other techniques to identify microorganisms, including microscopy, adenosine triphosphate photometry, hydrogenase measurements, adenosine phosphosulfate reductase, 4′,6-diamidino-2-phenylin-dole (DAPI), and MMM. MMM include qPCR, fluorescence in situ hybridization (FISH), denaturing gradient gel electrophoresis (DGGE), and clone library building. The advantages and disadvantages for each test have been described in detail elsewhere [18, 69, 70]. For example, MMM require sample preparation/fixation, the correct use of probes, and DNA extraction for accurate identification and quantification of microorganisms. Furthermore, the contribution of DNA from dead cells to the total DNA cannot be quantified. TM0212-2018 stresses the need to collect microbiological, operational, and chemical data from corroded sites and to compare with similar types of data collected from areas that are not corroded. Alabbas et al. [71] reported that DGGE was an ineffective method for fingerprinting DNA, specifically DNA from sour crude oil and seawater injection pipelines, because it is difficult to reproduce among different users and the information is visual, that is, there are no databases for comparative purposes. Investigators have used other approaches to describe microbial populations in petroleum reservoirs. Guan et al. [72, 73] used phylogenetic analyses of gene fragments of the dissimilatory sulfite reductase (DSR) gene that encodes for the key enzymes in the anaerobic dissimilatory reduction of sulfate. Their investigation demonstrated the diversity of SPP that could potentially be involved in reservoir souring and corrosion. Sequencing can provide the order of nucleotides in DNA or RNA. DNA sequencing can be used to determine the sequence of individual genes, gene clusters, chromosomes, or full genomes. Wang et al. [74] compared the results from pyrosequencing data and clone library searches to estimate bacterial diversity in aqueous and oil phases from a water-flooded petroleum reservoir. Pyrosequencing is a method that involves extracting DNA, suspending it in a fluid, breaking it apart using chemiluminescent enzymatic reactions, and using a high-resolution camera to infer its makeup. In molecular biology, a library is a collection of DNA fragments. The term can refer to a population of organisms. Using both pyrosequencing and clone library approaches, Wang et al. [74] determined that at a high phylogenic level, the predominant bacteria detected by the two methods were identical. However, they reported, “… pyrosequencing allowed the detection of more rare bacterial species than the clone library method.” Many techniques claim to monitor MIC; however, none has been accepted as an O&G industry standard or as a RP by ASTM or AMPP. NACE International TM0194-2014 “Field Monitoring of Bacterial Growth in Oil and Gas Systems” [56] describes a test method for monitoring growth of microorganisms and evaluating the effectiveness of control chemicals but does not relate directly to corrosion. The major limitation for MIC monitoring programs has been the inability to relate microorganisms to corrosion in real time. Some techniques can detect a specific modification in the system due to the presence and activities of microorganisms (e.g., heat transfer resistance, fluid friction resistance, and galvanic current) and assume something about the corrosion. Others measure some electrochemical parameter (e.g., polarization resistance, electrochemical noise) and assume something about microbial activities. Either experience or knowledge of a particular operating system can be an effective monitoring tool, especially for evaluating treatment regimes (biocides or corrosion inhibitors). There are approaches to derive real-time microbiological data. Chattoraj et al. [75] suggested that fluorogenic bioreporters, i.e., compounds that undergo a change in fluorescent signal after interaction with microorganisms, could be used to determine total microbial contamination/activity on-line and in real time. Chattoraj et al. [75] demonstrated that fluorescence, monitored with a fluorometer, before and after addition of the bioreporter, provided a ratio related to microbial activity. Several investigators have attempted to simultaneously quantify corrosion and some property related to microorganisms. For example, Haile et al. [76] developed a four-probe sensor for simultaneously monitoring corrosion rate and sulfide oxidase to detect sulfides. The probe has been demonstrated in the laboratory. The application of MMM for monitoring microbial populations has been suggested [77, 78]. Hoffman et al. [79] concluded, “… there are no ‘off the shelf’ solutions and standardized methods will ultimately be required if comparative data are to be generated across the industry.” Wolodko et al. [4] reviewed the models of MIC for the O&G industry stressing the need for more advanced microbiological detection and characterization. Predictive models of MIC in O&G carbon steel pipelines have remained unreliable because of uncertainties in the time to pit initiation and the rate of propagation. There have been attempts to predict MIC based on corrosivity factors. For example, Pots et al. [80] considered SRB the major contributor to MIC and determined that the following parameters influenced SRB activity: water, pH of the water, salinity, temperature, and nutrients, e.g., sulfate, total carbon, nitrogen, and carbon:nitrogen ratios. Each parameter was given a rating factor based on their influence. In addition, the operational history of the pipeline was reviewed, for example, duration of stagnation. Sooknah et al. [81, 82] used a similar approach to develop an internal pitting corrosion model that predicts susceptibility to MIC. Use of this type of model requires a thorough understanding of the specific system to which it is applied. Risk-based inspection programs that include MMM have been designed and are being tested [83, 84]. Larsen et al. [85] developed a model that estimates corrosion risk and time-before-pit initiation using qPCR enumeration of MIC-causing microorganisms and reverse transcript qPCR as a measure of cellular activity. Larsen et al. [85] used the approach during an inspection of two pipelines in the North Sea to develop a strategy for remediation. Standard mitigation procedures for controlling internal corrosion in O&G pipelines include physical removal of deposits (pigging) and chemical treatments that include nonoxidizing biocides (e.g., glutaraldehyde, quaternary amines, and tetrakis (hydroxymethyl) phosphonium sulfate). Biocides kill or slow the growth of microorganisms by a number of mechanisms, e.g., cross-linking proteins disrupting cell membranes, or inhibiting a vital process (e.g., synthesis or respiration) [86]. Maxwell and Campbell [87] used the approach developed by Pots et al. [80] described earlier to predict the risk of MIC in oil transportation lines. They [87] concluded, “… frequency of pigging—which will have no biocidal effect—is predicted to provide the greatest mitigation of MIC compared to the possible bacteriostatic effect of high salinity and the bactericidal effect of biocide additions.” Removal of surface deposits does introduce some risk, i.e., removal of protective oxides and dispersion of potentially corrosive microorganisms to other pipe sections [13]. Harris et al. [88] used the maximum pitting rate to evaluate the impact of film-forming corrosion inhibitors as a means of controlling MIC of carbon steel in produced waters. Film forming inhibitors (e.g., quaternary amines) are frequently used to control CO2 corrosion and can be used in combination with biocides (e.g., glutaraldehyde) intended to reduce numbers of microorganisms. In addition, some corrosion inhibitors contain toxic components. Harris et al. [88] indicated the following: (1) MIC pitting rates increased in the presence of some corrosion inhibitors, presumably because of the biodegradability of the inhibitor, (2) some microorganisms developed a resistance to supposedly toxic inhibitors, (3) MIC control was not related to toxicity, that is, a less toxic inhibitor provided better MIC control than a more toxic one, and (4) severe corrosion was not related to numbers of SRB. Similarly, Campbell et al. [89] suggested that evaluating MIC control or mitigation by monitoring a decrease in bacterial numbers is inaccurate and misleading. In their experiments, they concluded that biocides can injure cells in a biofilm, rather than killing the cells. Recovering SRB had significantly reduced doubling times compared with SRB that had not been exposed to biocides. Manipulation of electron acceptors can be used to stimulate or retard the growth of specific microbial populations. Both removal and addition of electron acceptors have been used to control microbial populations and MIC in seawater injection systems where seawater is injected into oil reservoirs to maintain pressure. In these applications, oxygen is removed to minimize corrosion. However, in the anaerobic environment, growth of SRB is encouraged. The concentration of sulfate in seawater is typically >2.0 g/l. Rizk et al. [90] used nanofiltration to reduce sulfate in seawater from 2.6 to 0.05 g/l. In laboratory studies, they demonstrated that the amount of H2S in the seawater was a direct function of the amount of sulfate in the water. The authors discussed the implication for corrosion but did not make corrosion measurements. In contrast, Jhobalia et al. [59] demonstrated that high-sulfate concentration in a laboratory medium (increase from 1.93 to 6.5 g/l) could inhibit the growth of Desulfovibrio desulfuricans and the corrosion rate of mild steel. The authors hypothesized that the observation was due to increasing toxicity of sulfate toward SRB metabolism or sulfate reduction. Addition of sulfate was presented as a “biochemical approach” for dealing with MIC [59] but has not been tested as a practical control strategy for MIC. Laboratory and field experiments have demonstrated that nitrate, an alternative to sulfate as an electron acceptor, addition can be an effective replacement for biocide treatment to reduce the sulfide production by SRB [91, 92], a process known as biocompetitive exclusion. The addition of nitrate can induce a shift in the dominant population from SRB to nitrate-reducing bacteria (NRB). NRB reduce nitrate to N2 with several possible intermediate by-products, including nitrite and ammonium. One motivation for nitrate injection is to prevent souring due to SRB. The other objective is to reduce corrosion risks. Nitrate treatment was implemented on an oil platform in the North Sea (Veslefrikk) [93]. The change from glutaraldehyde treatment to nitrate resulted in a dramatic change in the bacterial community. The SRB population decreased and the numbers of NRB increased. After 4 months of nitrate addition, the activity of SRB in the biofilm was markedly reduced as measured with radiorespirometry and an enrichment of NRB was measured. After 32 months of nitrate treatment, SRB numbers were reduced 20,000-fold and SRB activity was reduced 50-fold. Corrosion measurements decreased from 0.7 to 0.2 mm/year. Similar applications have been made to reduce souring [94, 95]. Gullfaks platforms have been treated with nitrate to reduce H2S production [96]. The authors also observed a 1000-fold reduction in SRB numbers and a 10- to 20-fold reduction in sulfate respiration activity and a 50% reduction in corrosion. Voordouw et al. [97] and Hubert et al. [98] demonstrated a nitrate-reducing, sulfide-oxidizing bacterium capable of reducing nitrate to nitrite, nitrous oxide, or nitrogen and oxidizing sulfide to sulfate or sulfur. The stoichiometry of the reactions catalyzed by the organism depended on the ratio of sulfide to nitrate. Dunsmore et al. [99] isolated an organism from a Danish North Sea oilfield water injection system that had been continuously treated with nitrate since the start of the injection. The organism could reduce nitrate and produce ammonium in the presence of sulfate, increasing the likelihood of corrosion. Hubert et al. [100] demonstrated that both nitrate and nitrite were effective treatments for decreasing sulfide concentrations. The required dose depended on the concentration of organics. Sunde et al. [96] suggested that reservoir characteristics and nutrient availability have a significant impact on the effectiveness of nitrate injection. There are several potential mechanisms for the observed inhibition of SRB due to the addition of nitrate. Microbial nitrate reduction produces more energy than sulfate reduction. It follows that if both nitrate and sulfate are present, nitrate will be the preferred electron acceptor, meaning that NRB can out-compete SRB in carbon-limited waters. In addition, reaction products from the reduction of nitrate to N2, e.g., nitrite, may inhibit SRB. A shift in the redox potential in the system may also inhibit SRB. Because of nitrate reduction, the redox potential will likely increase, producing unfavorable conditions for sulfate reduction. Nitrite may also act as a scavenger for H2S. The success of nitrate addition relies on a population of NRB in the system. Hubert et al. [98] suggested that bioaugmentation, in which ex situ grown microorganisms could be injected with nitrate if indigenous NRB were lacking. Despite the possibility of bioaugmentation, there are several reports of failures. Bouchez et al. [101] attempted to inoculate a nitrifying sequencing batch reactor with an aerobic denitrifying bacterium. The added bacterium disappeared after 2 days. Similarly, Hubert et al. [100] reported that the introduction of microorganisms into natural communities was difficult. Microorganisms other than SRB and NRB may be involved in subterranean nitrogen cycling. For example, in an anaerobic environment, ammonium-oxidizing (anammox) bacteria can convert ammonium and nitrite into N2. Li et al. [102] identified five genera of anammox bacteria in high-temperature petroleum reservoirs. In summary, nitrate addition and sulfate removal/reduction both attempt to control MIC caused by SRB. The long-term consequences on the microbial populations and MIC are unknown. There are limited data indicating that nitrate injection is ineffective at slowing H2S production where souring has already occurred [103]. Nitrate injection in sour systems can stimulate microorganisms to couple nitrate reduction to sulfur oxidation, resulting in additional corrosive sulfur species, e.g., elemental sulfur, polysulfides and thiosulfate [104]. Ciaraldi et al. [31] described the following tactics to prevent MIC in oil production lines and equipment: Subsurface environments are biologically active. Consequently, microorganisms and microbial activity can be anticipated on the surfaces of buried and submerged pipelines and under some circumstances can influence corrosion. As is the case with internal corrosion, the potential for MIC impacting the outer surfaces of buried or submerged pipelines is controlled by availability of water, electron acceptors/donors, and nutrients in the environment. Baker and Fessler [105] reported, “although salt water is more corrosive than most soil environments, cases of significant external corrosion on offshore pipelines are extremely rare.” The report concludes that control of external corrosion in the offshore environment has been “mastered” because of the homogeneity of the offshore environment. Steel pipelines for many industries, e.g., water distribution systems can be bare, uncoated or otherwise protected. Most O&G pipelines are coated and cathodically protected [106]. However, some uncoated steel lines are in O&G service. Prior to 49CFR Part 192 (1970) distribution pipes were not required to have corrosion protection, i.e., coatings or cathodic protection (CP). Consequently, older distribution systems may contain miles of pipe that are unprotected [107]. It is important to make that distinction when addressing external corrosion, in general, and MIC specifically. Wasim and Djukic [108] reviewed failure mechanisms for buried natural gas and oil pipelines, i.e., hydrogen-induced cracking, hydrogen embrittlement, corrosion fatigue, stress corrosion cracking, and MIC. The authors provide useful references on these mechanisms and conclude “… 20–30% of the external corrosion is reported to be due to MIC for buried oil and gas pipelines.” Spark et al. [109] suggested that the mechanisms for external corrosion of uncoated pipelines are largely independent of the pipe contents, e.g., water and crude oil. Their review of corrosion for buried uncoated steel provides a discussion of causative microorganisms and mechanisms. A long-term corrosion study (1922–1940) of buried uncoated steel was conducted by the National Bureau of Standards (NBS) renamed the National Institute of Standards (NIST) in 1988. Pipe samples were buried at 47 locations, representing a range soil types and climates throughout the United States. The study was designed to determine whether coatings were required to prevent corrosion in soil environments and if soil properties could be used to predict corrosivity. The resulting data indicated that corrosion rates typically attenuated after the first years of exposure. Romanoff [110] identified aeration, pH, concentration of soluble salts chemical as significant parameters related to corrosion. Importantly, Romanoff [110] concluded that soil resistivity correlated with corrosion and was a useful measure of the concentration of dissolved salts. Ricker [111] published a detailed analysis of the original Romanoff [110] data. Despite criticisms of the NBS study, e.g., limited characterization of soils, varied burial depths and exposure times between sites, averaged climatic conditions, and disregard for moisture, e.g., rainfall, Romanoff data and conclusions are still used to characterize the corrosivity of soils [112]. The NBS study did not consider the relationship between soil characteristics and microorganisms. The role of microorganisms in the corrosion of uncoated steel pipelines corrosion remains controversial. For example, Melchers and Petersen [113] reinterpreted the Romanoff NBS data and summarized the following findings. They agreed with Romanoff that corrosion rates were faster in the first few years of exposure compared to later years. Rates were highest for clays and calcareous soils and those with grit or gravel. Corrosion was less aggressive in silt or clay loams and rates were lowest in well-drained sands and sandy loams. However, Melchers and Petersen [113] concluded that the chemical properties of native soils had no “discernible effect on corrosion” of steels in soils. Instead, Melchers and Petersen [113] investigating uncoated steel pipe in drinking water distribution systems, suggested that the properties of the backfill were controlling corrosion, e.g., air pore space, internal drainage, and time-of-wetness (TOW). Melchers and Wells [114] concluded the following. (1) Soil resistivity could not be correlated with corrosion of steels, i.e., soil resistivity is not an indicator of corrosivity. (2) Melchers and Petersen [113] concluded there was no general relationship between uniform and localized corrosion, i.e., pit depth. The authors maintained that corrosion of buried steel is the result of localized differential aeration. Melchers [115] hypothesized that the numbers and types of microorganisms and therefore MIC on buried pipe surfaces might be limited by nutrient availability. In contrast, Usher et al. [116] provided references suggesting that soil holds the most biodiversity the largest populations of microorganisms in any habitat on Earth. A gram of soil may contain 1010 bacteria (not including fungi and archaea). Kieft [117] estimated that only 0.001–4% of soil microorganisms could be cultured on organic growth media. Peabody [118] indicated that soil moisture and numbers of bacteria were greater in back fill material than in undisturbed soil adjacent to a pipeline. Backfill was less consolidated and allowed greater penetration of moisture and oxygen. In recent reviews, Usher et al. [116] and Spark et al. [109] reviewed MIC for buried carbon steel pipelines. Usher et al. [116] presented a discussion of soil characteristics, microorganisms, and potential mechanisms for MIC of uncoated carbon steel assets. One of the earliest reports of MIC [119] identified SRB as the cause of external corrosion of iron pipe failures in sulfate-rich soils. SRB are still the organisms that are most frequently cited as causing external corrosion on pipe surfaces. The techniques used to detect, monitor, and diagnose MIC on buried pipe surfaces are essentially the same as those described in the previous section on internal pipe MIC and will not be described in detail. NACE International TM0106-2016 “Detection, Testing, and Evaluation of MIC on External Surfaces of Buried Pipelines” [120] is meant to identify MIC after it has occurred and does not describe a predictive methodology. In all cases, the testing requires identification and quantification of microorganisms associated with either soil from the burial location or surface deposits [121]. Importantly, the observation that planktonic microorganisms cannot be used to predict numbers and types of surface-related microorganisms in aqueous environments is also true for bulk soil microorganisms and those in biofilms. Grzelak [122] performed a comprehensive statistical analysis of corrosion rates and environmental parameters for MIC on external pipe surfaces, including dissolved oxygen concentration, redox potential, conductivity, pH, total organic carbon, SRB concentration, water level, and pipe-to-soil potential. Analysis of the corrosion products and the appearance of pitting on buried high-pressure gas transportation pipelines led to the conclusion that the corrosion was influenced by SRB. The author demonstrated weak positive correlations between individual parameters. However, the corrosion rate was more strongly influenced by interactions of parameters. Jack et al. [123] reported that MIC was responsible for 27% of all corrosion deposits on the exterior of line pipe in a survey of Nova Gas Transmission Ltd. (Calgary, Alberta) pipelines, where line pipe is defined by the American Petroleum Institute (API) as dimensional pipe sections, less fittings, and flanges. In a review of mechanisms for SRB influenced corrosion, Enning and Garrelfs [124] provided images of localized corrosion on the exterior of a buried carbon steel (unspecified) pipe exhumed from a water-logged, anoxic, sulfate-rich soil (Figure 55.2), illustrating pit morphology. Figure 55.2 (a) External corrosion on buried gas transmission pipeline excavated from a water-logged, anoxic, sulfate-rich soil. (b and c) Details of corrosion under a disbonded asphalt coating, illustrating clusters of pits and pits within pits. Numbers in B denote pit depths in mm. Bar = 2 cm. (Reference [124]/with permission of American Society for Microbiology.) In laboratory experiments, Chen et al. [125] demonstrated that the numbers, sizes, and depths of pits in X80 pipeline steel significantly increased in inoculated (Desulfovibrio desulfuricans) soil suspensions, compared to pitting in sterile soil suspensions. In the absence of SRB, the maximum pitting depth was 2.32 ± 0.2 μm, while the maximum depth in the inoculated sample was 6.01 ± 0.6 μm. In addition, some pits in inoculated samples were connected to form clusters. However, the pit morphologies, observed after 14-day exposures were the same, i.e., multiple tiers. Most investigators agree that although certain pit morphologies are consistent with some microbially influenced chemistries, morphology of localized corrosion cannot be used to diagnose MIC [126, 127]. While detailed information has been collected on the specifics of corrosion of buried pipeline corrosion, this has not resulted in a comprehensive understanding or model of corrosion in soils [128]. 49CFR Part 192 (1970) requires line pipe to be externally coated (e.g., asphalts, polyolefin tapes, and fusion bonded epoxies) and cathodically protected. Coatings isolate the pipe exterior surface from the environment. CP is the application of current to a pipeline, overriding local anodes and making the entire pipeline surface a cathode. Coatings reduce the exposed area of pipeline surfaces, making CP economically feasible. NACE International Standard Practice (SP) SP0169-2013 (formerly RP0169-2002) “Control of External Corrosion on Underground or Submerged Metallic Piping Systems” [129] describes the “… procedures and practices for achieving effective control of external corrosion …” The SP lists the following conditions in which CP is ineffective “elevated temperature, disbonded coatings, thermal insulating coatings, shielding, bacterial attack, and unusual contaminants in the electrolyte.” Microorganisms can affect CP in several ways. Some microorganisms are attracted to the areas surrounding a cathodically polarized pipe, that is, microbial abundance, activity and diversity in saturated soils, seawater or sediments could increase because of CP. If microorganisms compromise the coating, the potential required to prevent corrosion becomes more negative than the –850 mV versus saturated copper/copper sulfate reference electrode recommended in SP0169-2007. Barlo and Berry [130] confirmed that the criterion for CP of buried pipelines was valid in concept; however, the actual protection potential varied with environment. Microorganisms can increase the kinetics of corrosion reactions, necessitating a current increase to maintain a specific potential. When sufficient CP levels exist, corrosion is mitigated even in the presence of bacteria. The difficulty is determining the adequate protection potential. Microorganisms can degrade adhesives and coatings exposing metal. Pope and Morris [131] indicated that almost all cases of MIC on external surfaces were associated with disbonded coatings or areas shielded from CP. Abedi et al. [132] reported the failure of an oil transmission line due to external corrosion. In their study, a polyethylene tape coating on the exterior of the pipeline became loose, exposing the pipe surface to wet soil. The failure was attributed to SRB and stress corrosion cracking. With adequate CP fusion bonded epoxy coatings allow for CP of the pipe surface should the coating bond fail [133] because they do not shield the cathodic current. Published conclusions regarding MIC of O&G assets must provide more information than the location of the corrosion, i.e., internal or external corrosion. Statistics for internal corrosion should be accompanied by a description of pipe contents and operating conditions. Statistics for external corrosion should provide details about the soil conditions and a description of the pipe, e.g., coated, uncoated, CP protected, or disbonded coating. Literature on MIC of internal and external O&G assets is dominated by identification and enumeration of bacteria, particularly SRB. The weaknesses of this approach is that microorganisms other than bacteria can produce corrosive sulfides and sulfate is not the only electron acceptor that can be reduced to sulfide. Most importantly, numbers of SRB cannot be used to predict or diagnose MIC. Researchers investigating external corrosion cite the interaction of multiple environmental parameters as strongly correlated to corrosion rate while the concentration of SRB was not. In internal corrosion studies, investigators suggest an integrated study of corrosion chemistry and kinetics by metatranscriptomic analysis might lead to a better understanding of the interaction between microbial activities and redox chemistry involved in MIC. Much of the mechanistic information related to MIC and O&G pipelines is based on identification and enumeration of specific groups of bacteria and laboratory exposures that are typified by short exposure times, artificial media, and pure cultures. Microorganisms in biofilms demonstrate metabolic versatility based on electron acceptors and nutrients in the environment that cannot be approximated in the laboratory. Careful examination of samples from the field has provided new insights into the microbial diversity associated with MIC and the potential for microbial interactions that were not previously appreciated. Current MIC corrosion risk assessment models attempt to assign corrosivity factors to operational and environmental parameters. The weight attributed to each factor is arbitrary and requires a complete understanding of the system. Much of the information included in this chapter relates to processes that have been evaluated at 40 °C or less. The present standards and methodologies will not be adequate for evaluation of MIC in deeper, hotter reservoirs. Future testing may require different approaches (i.e., higher pressure, higher temperature, and flow) and new standards to measure and monitor MIC.
55
Microbiologically Influenced Corrosion
55.1 Introduction
55.2 Materials
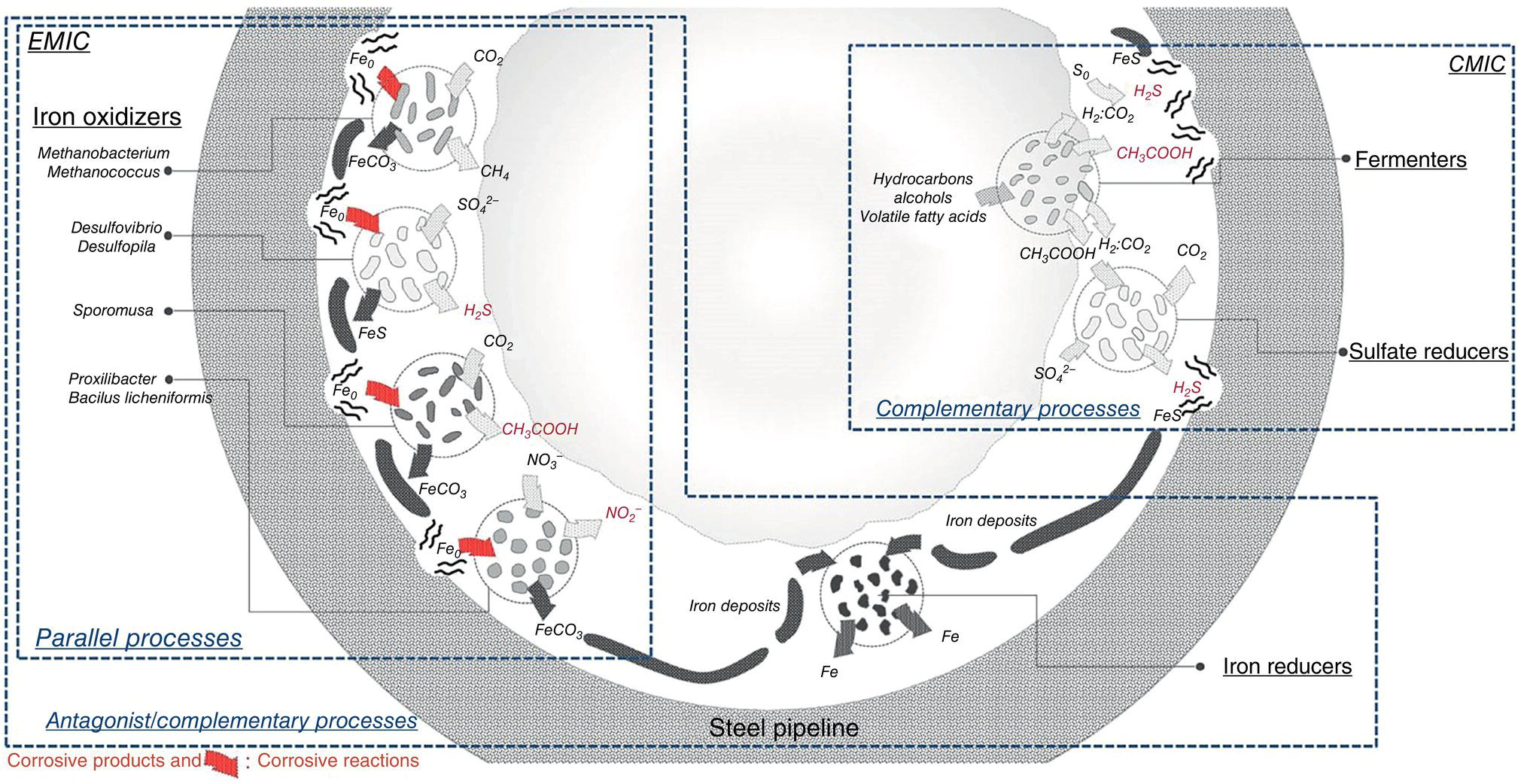
55.3 Microorganisms
55.3.1 Water
55.3.2 Electron Donors and Acceptors
55.3.3 Nutrients
55.4 Internal Corrosion
55.4.1 Types of Pipelines
55.4.1.1 Production and Gathering Pipelines
55.4.1.2 Transmission Lines
55.4.1.3 Distribution and Storage
Gasoline
Aviation Fuel
Diesel
55.4.2 Detection, Monitoring, and Diagnosing
55.4.3 Modeling
55.4.4 Control
55.4.4.1 Reduce Surface-Associated Microorganisms
55.4.4.2 Manipulation of Electron Acceptor
55.4.4.3 Best Practices to Prevent Internal MIC in O&G Pipelines
55.5 External Corrosion
55.5.1 Types of Pipelines
55.5.1.1 Submerged Pipelines
55.5.1.2 Buried Pipelines
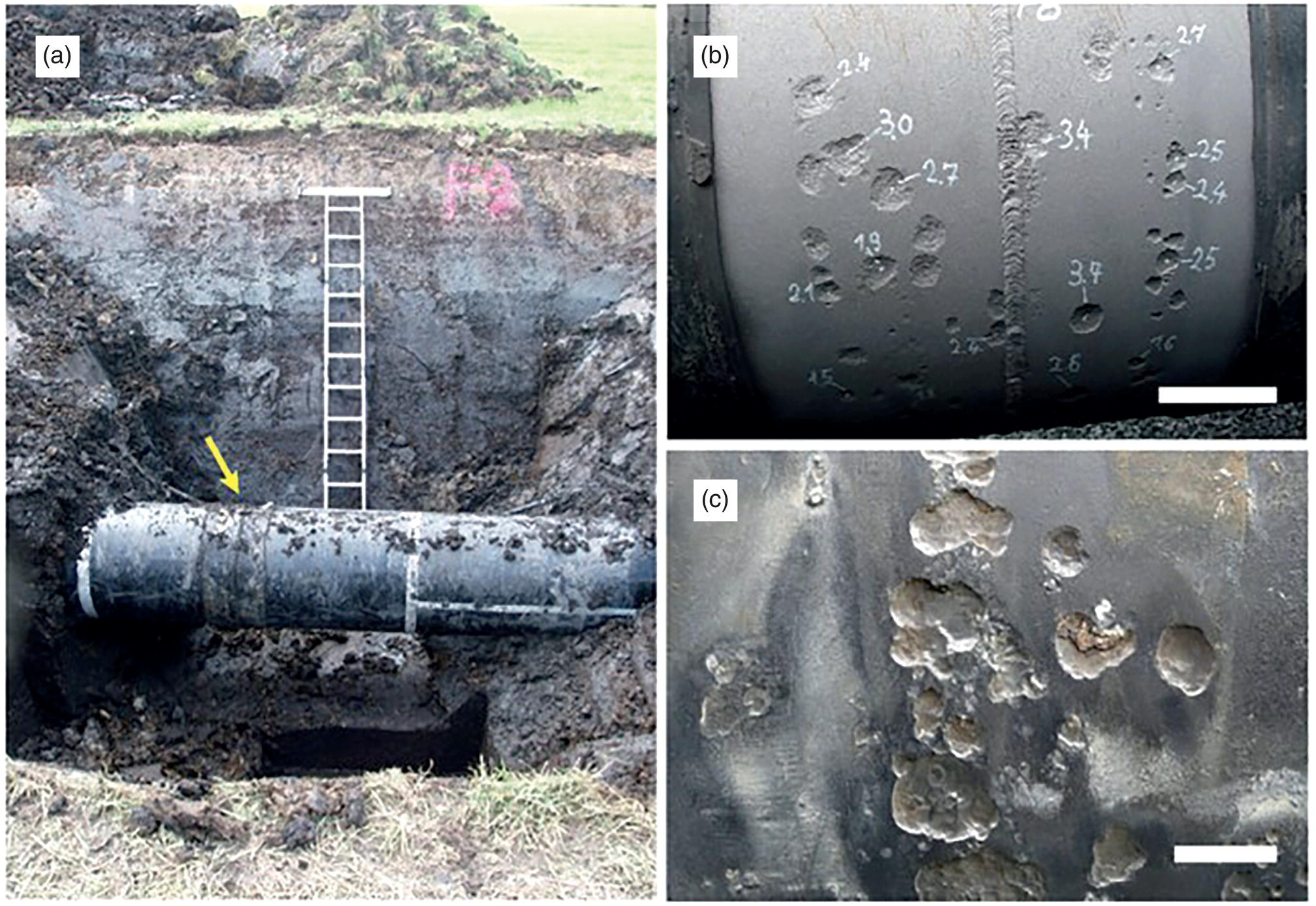
55.5.2 Detection, Monitoring and Diagnosing
55.5.3 Modeling
55.5.4 Control
55.5.5 Conclusions
References